Food Commercial Sterility Testing Method and Analysis
Published date and time: 2020-09-08, 16:12
Date and time of modification: 2021-05-02, 10:18
Author: Food-Men
Commercial sterility tests are essential for food of cans, glass bottles and canned soft containers. This article introduces food commercial sterility testing method and abnormal causes analysis to meet FDA requirements.
1. Description of Some Terms
1.1 Commercial Sterility
Commercial sterility means no pathogenic bacteria and toxins that endanger public health; does not contain any microorganisms that can be propagated during storage, transportation and sale of the product; maintains stable quality and good commercial value during the life of the product.
1.2 Low Acid Canned Food
In addition to alcoholic beverages, canned foods with a balanced pH greater than 4.6 and a water activity greater than 0.85. For example: low-acid fruit, vegetable or vegetable products. Those which add acid to lower the pH for the purpose of heat sterilization are acidified low-acid canned foods.
1.3 Acid Canned Food
Acidic canned food refers to a canned food with an equilibrium pH of 4.6 or less after sterilization. For example: tomatoes, pears, pineapples and juices made from them having a pH of less than 4.6.
1.4 Type of Can Container
The types of can containers mainly include metal cans (tin cans, aluminum cans), glass cans (bottles), plastic cans (containers of various shapes), and containers made of composite materials.
2. Commercial Sterility Testing Process
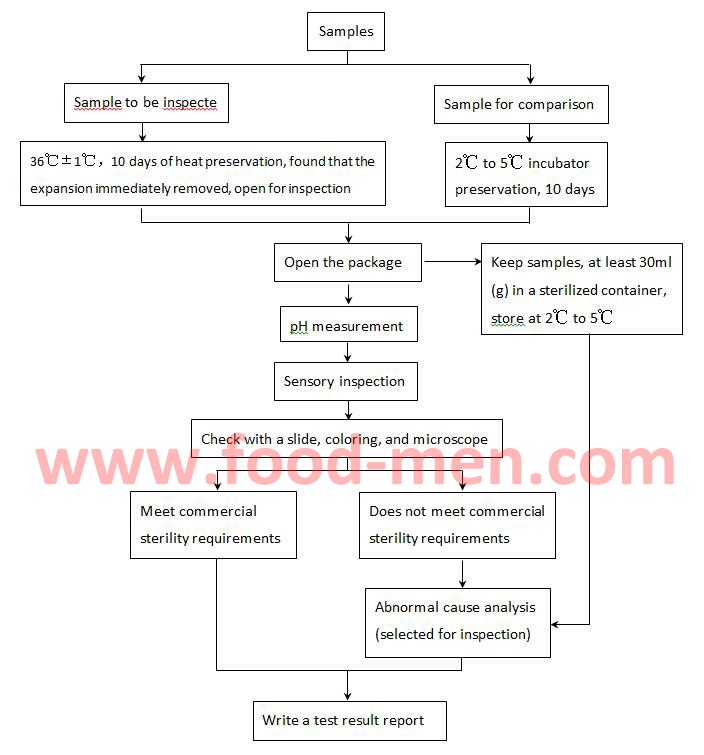
3. Instructions of Wperation for Commercial Sterility Testing
(Click on the blue font to browse another page to view the relevant introduction)
3.1 All tools that contact the contents of the sample should be sterilized by Horizontal Laboratory Autoclave Sterilizers or Vertical Laboratory Autoclave Sterilizers.
3.2 The operation of the sample opening or after opening the lid shall be carried out in an air-cleaned environment, that is aseptic operation. The aseptic operation can be performed in a sterile laboratory (clean room) or clean benches.
3.3 If the contents are a mixture of solid and liquid, put them in Sterile Sample Blender Bags, seal the bags, pat with stomacher blenders, break the solids of the contents, and then inspect.
3.4 If the contents are multiple liquids and you want to stir evenly, use a Magnetic Stirrer Mixer with Timer and HotPlate or a 4 Heads Magnetic Stirrer Mixer with HotPlate or Multifunctional Orbital Shaker Incubators for Laboratory, to ensure that there are no contaminated by microorganisms when are stirring.
3.5 Sensory inspection refers to observing the color and tissue morphology of the contents of the sample, and feeling their odor.
3.6 When the sample is found to be inconsistent with the requirements of commercial sterility, it is necessary to analyze the cause of the abnormality, and if necessary, special culture and detection of the bacteria to find out the cause.
3.7 A blank test is required (sample test of no microorganisms). When there is a microbe in the blank test, it indicates that there is a problem with the test operation and the result is not credible.
4. Abnormal Cause Analysis for Commercial Sterility Test
4.1 If no microorganisms are found in the expanded sample, this expansion may be due to a chemical reaction between the contents and the container. For example, acidic foods corrode with metals in metal containers and produce gases. In addition, overfilling the contents may also result in slight swelling.
4.2 Microscopic examination reveals a large number of bacteria, but does not grow after culture. This may be the corpse of bacteria, which may be the corruption that occurs before sterilization.
4.3 The packaging container is "sealed good", and there are microbes that are not heat-resistant under the culture condition of 36℃ (such as yeast), it may be leaking of the container or no sterilization during the production process.
4.4 The packaging container has good sealing performance. Only Bacillus grows under the condition of 36℃ culture, and their heat resistance is not higher than Clostridium botulinum, indicating insufficient sterilization.
4.5 Some anaerobic bacteria do not produce gas, there is no expansion of the packaging container, no leakage, only the surface of the container is difficult to find the bacteria of the contents, but the pH of the content is detected, the product becomes sour (acidified). The contents were inoculated in bromocresol purple glucose broth medium (test tube); cultured at 36℃ or 55℃, if bacteria were present, and acid was produced, indicating that there was a mesophilic microorganism. For example: flat-sour bacteria, Thermophilic Bacillus, and the like. If these bacteria appear, the bactericidal strength of the can should be increased.
The microorganisms are grown in different environments through different culture media; the morphology, gas production and acid production of the microorganisms are observed to find out the types of microorganisms. This will analyze the causes of food corruption and provide a basis for improving food production.
5. Methods of microbial staining observation
5.1 Crystal violet staining solution
5.1.1 Compositions
Crystal violet: 1.0g
95% Ethanol: 20.0mL
1% Ammonium oxalate solution: 80.0mL
5.1.2 Preparation method
Dissolve 1.0g of Crystal Violet in 95% ethanol, and then mix with 1% ammonium oxalate solution.
5.1.3 Staining method
Fix the smear with the flame from an alcohol burner. Add crystal violet to the smear drop by drop and stain for 1 min, then rinse with water.
5.2 Gram stain solution
5.2.1 Crystal violet staining solution
5.2.1.1 Compositions
Crystal violet: 1.0g
95% Ethanol: 20.0mL
1% Ammonium oxalate solution: 80.0mL
5.2.1.2 Preparation method
Dissolve 1.0g of crystal violet fully in 95% ethanol and then mix with 1% ammonium oxalate solution.
5.2.2 Gram’s iodine solution
5.2.2.1 Compositions
Iodine: 1.0g
Potassium iodide: 2.0g
Distilled water: 300mL
5.2.2.2 Preparation method
First, mix 1.0g of iodide with 2.0g of potassium iodide. Then add a little distilled water into the mixture and shake well. After it is fully dissolved, add distilled water to 300mL.
5.2.3 Safranine (counterstain)
5.2.3.1 Compositions
Safranine: 0.25g
95% Ethanol: 10.0mL
Distilled water: 90.0mL
5.2.3.2 Preparation Method
Dissolve 0.25g of safranine in ethanol and then dilute with distilled water.
5.2.4 Staining Method
5.2.4.1 Fix a smear on the flame from an alcohol burner. Add crystal violet to the smear drop by drop and stain for 1 min, then rinse with water.
5.2.4.2 Add gram’s iodine drop by drop, retain for 1 minute and then rinse with water.
5.2.4.3 Add 95% ethanol drop by drop, decolor for about 15~30s until the staining solution is washed off. Decoloring too much is not allowed, and then rinse with water.
5.2.4.4 Add counterstain liquid drop by drop, re-dye for 1 minute and rinse with water. Conduct microscopy after drying.
6. Preparation of culture medium and nutrient solution
6.1 Bromcresol purple dextrose broth
6.1.1 Compositions
Peptone: 10.0g
Beef extract: 3.0g
Glucose: 10.0g
Sodium chloride: 5.0g
Bromocresol Purple: 0.04g (or 1.6% ethanol solution, 2.0mL)
Distilled water 1000.0mL
6.1.2 Preparation method
Heat, mix and dissolve all the compositions excluding Bromocresol Purple. Adjust pH to 7.0±0.2 and then add Bromocresol Purple into the mixed solution. Dispense it into test tubes with small inversed test tubes. And each tube should be filed withl 10mL of the mixed solution and autoclaved at 121℃ for 10 minutes.
6.2 Cooked meat medium
6.2.1 Compositions
Beef infusion broth: 1000.0mL
Peptone: 30.0g
Yeast extract: 5.0g
Glucose: 3.0g
Sodium dihydrogen phosphate: 5.0g
Soluble starch: 2.0g
Meat residue: appropriate amount
6.2.2 Preparation method
6.2.2.1 Weigh 500g of fresh minced beef without fat and fascia, then mixed with 1000mL of distilled water and 25.0mL of 1moL/L sodium hydroxide solution. Stir the solution and boil it for 15 minutes, and cool it down. Remove the external fat, clarify and filter the solution. Add 1000mL of water to get the beef infusion broth. Add in all the compositions except meat residue referred to in 6.2.1 , and then adjust the pH value to 7.8±0.2.
6.2.2.2 Wash the meat residue with water and make it half-dry. Respectively fill it into test tubes (15mm×150mm) which is about 2cm to 3cm high. Add 0.1g to 0.2g reduced iron powder or a few iron filings into each test tube. Fill every test tube with the prepared liquid culture medium of 6.2.2.1, making the liquid about 1 cm higher than the surface of meat residue. It is then topped with dissolved petrolatum or liquid paraffin (0.3cm to 0.4cm high). Autoclave at 121℃ for 15 minutes.
6.3 Nutrient agar
6.3.1 Compositions
Peptone: 10.0g
Beef extract: 3.0g
Sodium chloride: 5.0g
Agar: 15.0g to 20.0g
Distilled water: 1000.0mL
6.3.2 Preparation method
Dissolve all the compositions except agar in distilled water, and then add in about 2mL of 15% sodium hydroxide solution. Adjust pH to 7.2 to 7.4. Then add in agar and heat to boiling to dissolve agar. Dispense the obtained solution into flasks or test tubes (13mm×130mm) respectively. Autoclave at 121℃ for 15 minutes.
6.4 Acid broth
6.4.1 Compositions
Polypetone: 5.0g
Yeast extract: 5.0g
Glucose: 5.0g
Potassium dihydrogen phosphate: 5.0g
Distilled water: 1000.0mL
6.4.2 Preparation method
Heat, stir and dissolve all the compositions referred to in 6.4.1. Adjust pH to 5.0±0.2. Autoclave at 121℃ for 15 minutes.
6.5 Malt extract broth
6.5.1 Compositions
Malt extract: 15.0g
Distilled water: 1000.0mL
6.5.2 Preparation method
Dissolve malt extract fully in distilled water, then filter by filter paper. Adjust pH to 4.7±0.2 and dispense it to several containers. Autoclave at 121℃ for 15 minutes.
6.6 Sabouraud's dextrose agar
6.6.1 Compositions
Peptone: 10.0g
Agar: 15.0g
Glucose: 40.0g
Distilled water: 1000.0mL
6.6.2 Preparation method
Dissolve all the compositions in distilled water and heat to boiling. Adjust pH to 5.6±0.2. Autoclave at 121℃ for 15 minutes.
6.7 Liver veal agar
6.7.1 Compositions
Liver extract: 50.0g
Veal extract: 500.0g
Proteose peptone: 20.0g
Neopeptone: 1.3g
Tryptone: 1.3g
Glucose: 5.0g
Soluble starch: 10.0g
Plasma casein: 2.0g
Sodium chloride: 5.0g
Sodium nitrate: 2.0g
Gelatin: 20.0g
Agar: 15.0g
Distilled water: 1000.0mL
6.7.2 Preparation method
Dissolve all the compositions in distilled water. Adjust pH to 7.3±0.2. Autoclave at 121℃ for 15 minutes.
Note
Most countries have provisions for commercial sterility testing of food, but the regulations for each country may vary. This article mainly refers to the relevant commercial sterility test specifications of the People's Republic of China.
——————————————————————
Related Articles
1. Food Commercial Sterility Testing and Equipment
2. Equipment of Aerobic Plate Count
3. Equipment for testing and counting coliforms
4. Canned Preservation Principle (1) - General Knowledge
5. Canned Preservation Principle (2) - Cans Sizes
6. Canned Preservation Principle (3) - 3-piece Cans Resistance Welding
7. Canned Preservation Principle (4) - Cans Double Seam Seal
8. Canned Preservation Principle (5) - The Generation of Vacuum in the Can
9. Canned Preservation Principle (6) - Sterilization of Canned Food
10. Method of Drinking Water Automatic Treatment and Purification
—————————————————————— 